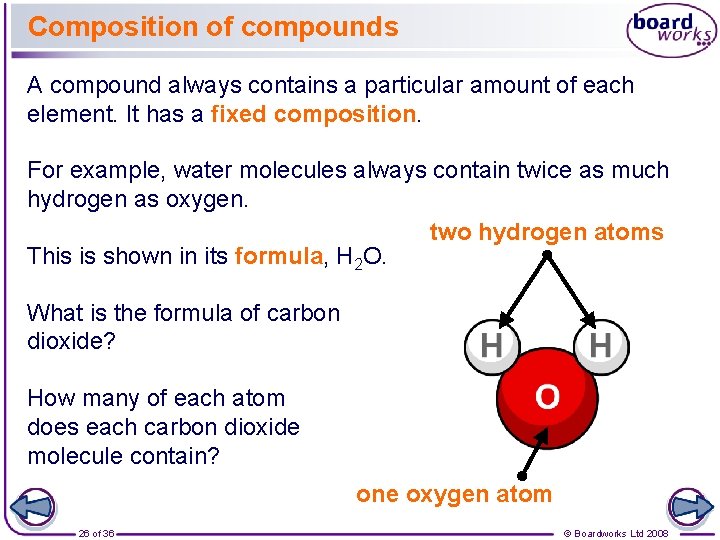
Twice as many hydrogen atoms as oxygen atoms.
If you’re searching for twice as many hydrogen atoms as oxygen atoms pictures information related to the twice as many hydrogen atoms as oxygen atoms interest, you have pay a visit to the ideal blog. Our site frequently provides you with hints for downloading the maximum quality video and picture content, please kindly surf and locate more enlightening video articles and graphics that match your interests.
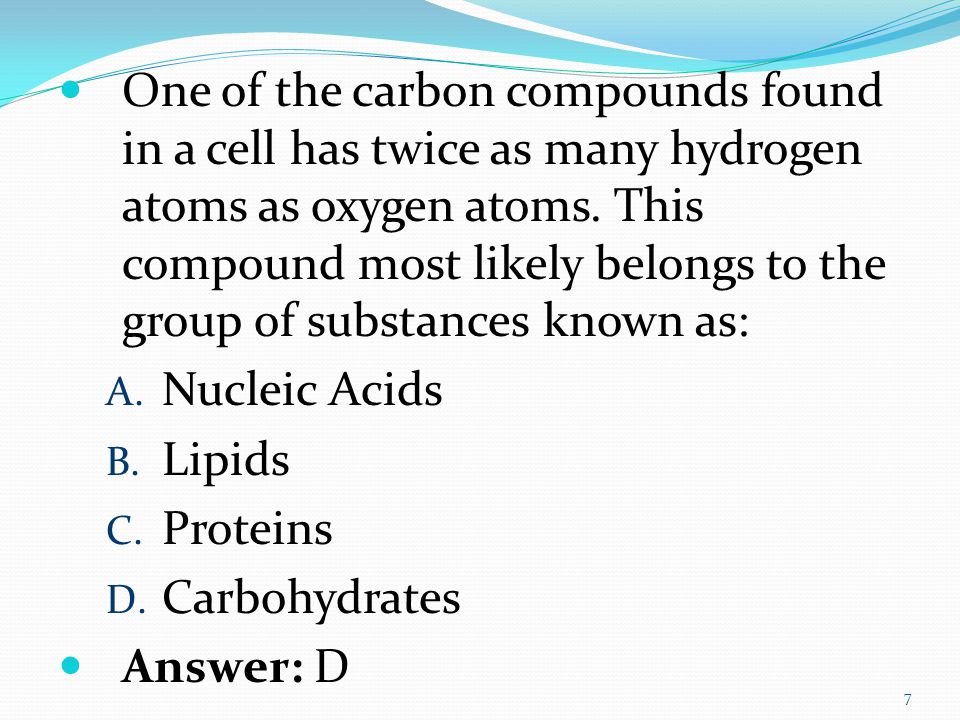 Twice As Many Hydrogen Atoms As Oxygen Atoms From twicemembersprofile.blogspot.com
Twice As Many Hydrogen Atoms As Oxygen Atoms From twicemembersprofile.blogspot.com
In the third option the compound has 2 hydrogen atoms and 1 Oxygen atom. There are ½ times as many oxygen atoms as hydrogen atoms. Its structural formula is pictured below. Algebra QA Library A ribose sugar molecule in RNA contains oxygencarbon and hydrogen.
In the third option the compound has 2 hydrogen atoms and 1 Oxygen atom.
Therefore there are twice as many oxygen atoms as hydrogen atoms. In the second option the compound has 2 hydrogen atom and 4 Oxygen atoms. Its structural formula is pictured below. The molar mass of the compound is 152 gmol. There are ½ times as many oxygen atoms as hydrogen atoms.

Carbohydrates- In carbohydrates the number of hydrogen atoms are twice the amount of oxygen atoms. In the fourth option the compound has 3 hydrogen. In the third option the compound has 2 hydrogen atoms and 1 Oxygen atom. There are twice as many hydrogen atoms as carbon atoms. A certain molecule contains twice as many atoms of hydrogen as oxygen and one more atom of carbon than hydrogen.
Thus the mass ratio is Thus the mass ratio is dfractextmass of 2 O atomstextmass of 2 H atoms dfractext2 x 15873 x mass of.
A good example of this is glucose C6H12O6. That is 24 12. How many oxygen atoms are present in 228 moles of nitric acid hno3. The amount of hydrogen atoms is two times larger than the oxygen.
 Source: slideplayer.com
Source: slideplayer.com
Therefore there are twice as many oxygen atoms as hydrogen atoms. The number of oxygen atoms equal the number of carbon atoms. Twice as many oxygen atoms correspond to twice the mass of oxygen. In this instance the symbol for hydrogen occurs twice once with a subscript of 4.
 Source: pinterest.com
Source: pinterest.com
A molecule of an organic compound has twice as many hydrogen atoms as carbon atoms the same number of oxygen atoms as carbon atoms and one-eighth as many sulfur atoms as hydrogen atoms. As you can see there are more than twice as many hydrogen atoms than oxygen. A molecule of an organic compound has twice as many hydrogen atoms as carbon atoms the same number of oxygen atoms as carbon atoms and one-eighth as many sulfur atoms as hydrogen atoms. There are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in this compound.

The compound contains Carbon Hydrogen and Oxygen atoms. In the third option the compound has 2 hydrogen atoms and 1 Oxygen atom. In the third option the compound has 2 hydrogen atoms and 1 Oxygen atom. The number of oxygen atoms equal the number of carbon atoms.
Solution The formula H 2 O 2 tells us that there are 2 oxygen atoms and 2 hydrogen atoms in each molecule. The molar mass of the compound is 152 gmol. For every oxygen atom two hydrogen atoms are needed. Thus the mass ratio is Thus the mass ratio is dfractextmass of 2 O atomstextmass of 2 H atoms dfractext2 x 15873 x mass of.
Estimate how much oxygen gas would be produced by and identical solution in 100s at 308 K hi can you please help me set up this problem then I.
The subscript in C6H12O6 indicates there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in this compound. Solution The formula H 2 O 2 tells us that there are 2 oxygen atoms and 2 hydrogen atoms in each molecule. A molecule of an organic compound has twice as many hydrogen atoms as carbon atoms the same number of oxygen atoms as carbon atoms and one-eighth as many sulfur atoms as hydrogen atoms. A certain molecule contains twice as many atoms of hydrogen as oxygen and one more atom of carbon than hydrogen. How many hydrogen atoms are present in 284 moles of water.

If the total number of atoms in the RNA molecule is 20. A molecule of an organic compound has twice as many hydrogen atoms as carbon atoms the same number of oxygen atoms as carbon atoms and one-eighth as many sulfur atoms as hydrogen atoms. How many oxygen atoms are present in 228 moles of nitric acid hno3. Estimate how much oxygen gas would be produced by and identical solution in 100s at 308 K hi can you please help me set up this problem then I. For the following fusion reaction Calculate change in energy per mole Answer in Jmole 2H1 2H1 — 3H1.
The number of hydrogen atoms is twice the number of oxygen atoms. How many hydrogen atoms are present in 284 moles of water. In which of these compounds are there twice as many oxygen atoms as hydrogen atoms. Normal oxygen has atomic mass 16 hydrogen 1 water has 1 oxygen and 2 hydrogen so the total molecular mass of water is 18.
For the following fusion reaction Calculate change in energy per mole Answer in Jmole 2H1 2H1 — 3H1.
As you can see there are more than twice as many hydrogen atoms than oxygen. There are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in this compound. Algebra QA Library A ribose sugar molecule in RNA contains oxygencarbon and hydrogen. Therefore there are twice as many oxygen atoms as hydrogen atoms.

The compound contains Carbon Hydrogen and Oxygen atoms. This mole of water has 6 x 1023 atoms of oxygen twice as many atoms of hydrogen. Therefore there are twice as many oxygen atoms as hydrogen atoms. Carbohydrates- In carbohydrates the number of hydrogen atoms are twice the amount of oxygen atoms.
 Source: everettcc.instructure.com
Source: everettcc.instructure.com
The number of oxygen atoms equal the number of carbon atoms. The molar mass of the compound is 152 gmol. Twice as many hydrogen atoms oxygen a certain molecule contains of. The molecular formula of glycerol is C3H8O3.
 Source: twicemembersprofile.blogspot.com
Source: twicemembersprofile.blogspot.com
In the second option the compound has 2 hydrogen atom and 4 Oxygen atoms. That is 24 12. A sugar molecule has twice as many atoms of hydrogen as it does oxygen and one more atom of carbon than of oxygen. That is 24 12.
This mole of water has 6 x 1023 atoms of oxygen twice as many atoms of hydrogen.
There are twice as many hydrogen atoms as carbon atoms. Solution The formula H 2 O 2 tells us that there are 2 oxygen atoms and 2 hydrogen atoms in each molecule. There are twice as many hydrogen atoms as carbon atoms. The molar mass of the compound is 152 gmol. One atom donates an electron to the other atom.
 Source: slidetodoc.com
Source: slidetodoc.com
Again using postulate 2 from Daltons Atomic Theory the atoms have characteristic masses and so a given number of hydrogen atoms corresponds to a fixed mass of hydrogen. Dicholromethanol has twice as many hydrogen atoms as oxygen atoms. Again using postulate 2 from Daltons Atomic Theory the atoms have characteristic masses and so a given number of hydrogen atoms corresponds to a fixed mass of hydrogen. If there are 21 atoms altogether in the molecule how many atoms of carbon are there. The compound contains Carbon Hydrogen and Oxygen atoms.
One atom donates an electron to the other atom.
Twice as many hydrogen atoms oxygen a certain molecule contains of. Carbohydrates- In carbohydrates the number of hydrogen atoms are twice the amount of oxygen atoms. In the second option the compound has 2 hydrogen atom and 4 Oxygen atoms. The amount of hydrogen atoms is two times larger than the oxygen.
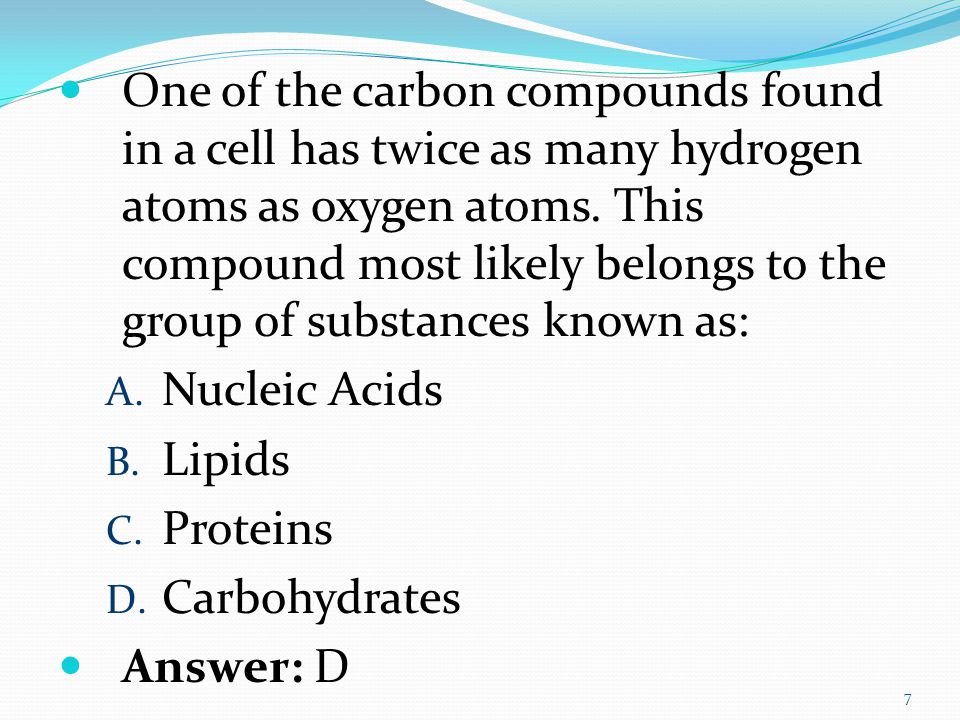 Source: twicemembersprofile.blogspot.com
Source: twicemembersprofile.blogspot.com
If the total number of atoms in the RNA molecule is 20. The number of hydrogen atoms is twice the number of oxygen atoms. In the third option the compound has 2 hydrogen atoms and 1 Oxygen atom. In the third option the compound has 2 hydrogen atoms and 1 Oxygen atom.
 Source: twicemembersprofile.blogspot.com
Source: twicemembersprofile.blogspot.com
Its structural formula is pictured below. Solution The formula H 2 O 2 tells us that there are 2 oxygen atoms and 2 hydrogen atoms in each molecule. Again using postulate 2 from Daltons Atomic Theory the atoms have characteristic masses and so a given number of hydrogen atoms corresponds to a fixed mass of hydrogen. There are ½ times as many oxygen atoms as hydrogen atoms.

A sugar molecule has twice as many atoms of hydrogen as it does oxygen and one more atom of carbon than of oxygen. 13966 results page 16 chemistry. The amount of hydrogen atoms is two times larger than the oxygen. How many oxygen atoms are present in 228 moles of nitric acid hno3 How many oxygen atoms are.
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Twice as many oxygen atoms correspond to twice the mass of oxygen. Thus the mass ratio is Thus the mass ratio is dfractextmass of 2 O atomstextmass of 2 H atoms dfractext2 x 15873 x mass of. Therefore there are twice as many oxygen atoms as hydrogen atoms. Twice as many oxygen atoms correspond to twice the mass of oxygen. The number of oxygen atoms equal the number of carbon atoms.
 Source: slidetodoc.com
Source: slidetodoc.com
The molar mass of the compound is 152 gmol. One atom donates an electron to the other atom. The compound contains Carbon Hydrogen and Oxygen atoms. How many are hydrogen. A molecule of an organic compound has twice as many hydrogen atoms as carbon atoms the same number of oxygen atoms as carbon atoms and one-eighth as many sulfur atoms as hydrogen atoms.
A molecule of an organic compound has twice as many hydrogen atoms as carbon atoms the same number of oxygen atoms as carbon atoms and one-eighth as many sulfur atoms as hydrogen atoms.
For every oxygen atom two hydrogen atoms are needed. In the third option the compound has 2 hydrogen atoms and 1 Oxygen atom. Dicholromethanol has twice as many hydrogen atoms as oxygen atoms. A good example of this is glucose C6H12O6.
 Source: slideplayer.com
Source: slideplayer.com
Carbohydrates- In carbohydrates the number of hydrogen atoms are twice the amount of oxygen atoms. The amount of hydrogen atoms is two times larger than the oxygen. Solution The formula H 2 O 2 tells us that there are 2 oxygen atoms and 2 hydrogen atoms in each molecule. That is 24 12. Twice as many oxygen atoms correspond to twice the mass of oxygen.

Its structural formula is pictured below. 13966 results page 16 chemistry. If a sugar molecule has a total of 45 atoms how many are oxygen. One atom donates an electron to the other atom. The number of hydrogen atoms is twice the number of oxygen atoms.

Therefore there are twice as many oxygen atoms as hydrogen atoms. In the second option the compound has 2 hydrogen atom and 4 Oxygen atoms. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. For every oxygen atom two hydrogen atoms are needed. 13966 results page 16 chemistry.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title twice as many hydrogen atoms as oxygen atoms by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





